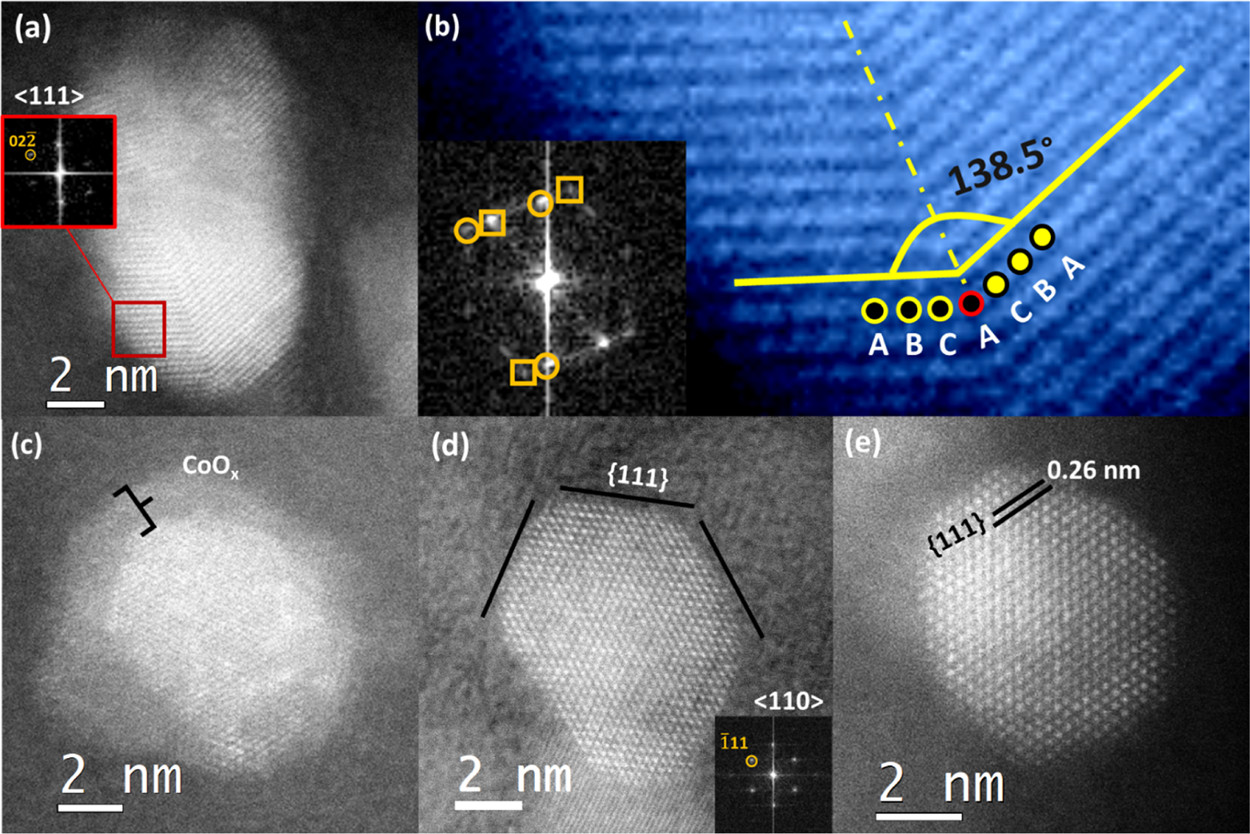
Scientists explore complex metal-support interactions under redox conditions using our Climate system
Via the DENSsolutions Climate system, a team of scientists uncover the dynamic interplay between platinum nanoparticles and titania support under reaction conditions.
Original article by H. Frey, A. Beck, X. Huang, J.A. van Bokhoven and M. G. Willinger

For catalysts consisting of metal nanoparticles and an oxide support, understanding the synergistic reactions between the metal and support is paramount. In the case of reducible supports, the so-called strong metal-support interaction (SMSI) provides a means of tuning the chemisorption and catalytic properties of supported metal particles. SMSI involves the encapsulation of nanoparticles by a thin layer of partially reduced support material. The encapsulation is typically induced during high-temperature reductive “activation”, i.e., treatment in hydrogen. Notably, the direct imaging of this encapsulated state has mostly been achieved ex situ. Little is known about SMSI under catalytic working conditions, where the application of in situ electron microscopy is invaluable. While environmental transmission electron microscopy (TEM) is generally limited to chamber pressures of around 20 mbar, the DENSsolutions Climate Nano-Reactor can handle pressures 100 times higher, unlocking unprecedented research possibilities for the study of catalysts in their native environment.
In recent research performed at ScopeM, ETH Zurich, where our advanced Climate G+ system is installed, Hannes Frey, Arik Beck, Dr. Xing Huang, Prof. Dr. Jeroen Anton van Bokhoven and Prof. Dr. Marc Georg Willinger investigated the dynamic interplay between metal nanoparticles and oxide support under reaction conditions. More specifically, the scientists revealed the working state of a model catalyst, Pt–TiO₂, by directly observing the synergistic interactions related to SMSI between the platinum nanoparticles and titania support.
Switching to a redox-active H₂–O₂ mixture
Frey and his fellow collaborators first induced the classical SMSI state by heating the titania-supported platinum nanoparticles (NPs) in H₂. The nanoparticles were then transferred into an O₂ atmosphere via inert gas purging. Interestingly, this treatment resulted in the platinum NPs incurring a nonclassical oxidized SMSI state. After preparing the system, the researchers then exposed it to a redox active atmosphere through the addition of H₂ into the O₂ flow. With the Climate G+ system, the researchers were able to mix the gasses on the fly, while continuing with the high-resolution observation.
In Figure 1 and Movie 1 below, the morphological change of the platinum NPs upon transition into the redox-active regime is presented. Increasing the partial pressure of H₂ in the Climate Nano-Reactor resulted in the gradual change in the encapsulated state of the Pt NPs. Moreover, this resulted in the ultimate disappearance of the overlayer as soon as the gas composition reached a set mixture of 60 mbar H₂ and 700 mbar O₂ after ~180 s. Once the overlayer was fully removed, the particle was observed to experience particle dynamics like restructuring and migration (shown in Figure 1E and 1F).

Figure 1: Image series depicting the morphological changes observed when a titania-supported platinum nanoparticle in a nonclassical SMSI state is exposed to a redox-active atmosphere. The composition of the gas in the Nano-Reactor was gradually changed from 700 mbar O₂ to a mixture of 60 mbar H₂ plus 700 mbar O₂. t0 is the time at which the H₂ flow was turned on.
Movie 1: TEM Movie depicting the morphological changes observed when a titania-supported platinum nanoparticle in a nonclassical SMSI state is exposed to a redox-active atmosphere.
Particle and interfacial dynamics in the redox-active regime
The researchers then set out to explore the response of a collection of nanoparticles to reaction conditions. They discovered that the degree of structural dynamics and mobility differed greatly among the nanoparticles. Whereas some NPs remained static and stationary, others incurred structural fluctuations and migrated across the substrate surface. The researchers decided to follow three representative cases of nanoparticles with different orientations. In all cases, it is observed that the redox chemistry at the interface is the driving force for particle reconstruction and migration. In Figure 2 below, the respective structural dynamics of the three selected nanoparticles are presented.

Figure 2: (A–C) Pt NP oriented with (111) planes perpendicular to the interface. (G to J) Pt NP oriented with (111) planes parallel to the interface. (K to N) Pt NP that has its (111) planes inclined toward the interface. The blue shapes indicate the respective positions of the Pt NP in the previous frames.
Case 1
The first platinum NP that was considered was oriented with (111) planes perpendicular to the interface. In Movie 2 below, the recorded time series of this platinum NP at 600°C in an atmosphere containing 700 mbar O₂ plus 60 mbar H₂ is shown. Here, the NP developed pronounced structural dynamics that involved twin formation and shearing along (111) planes in an up-down motion, perpendicular to the interface.
Movie 2: Recorded time series of the Pt NP presented in Figure 2A–C) at 600°C in an atmosphere containing 700 mbar O₂ plus 60 mbar H₂.
Case 2
The second Pt NP that was considered was oriented with (111) planes parallel to the interface. Movie 3 below shows the image series acquired for this nanoparticle, at 600°C in an atmosphere containing 700 mbar O₂ plus 60 mbar H₂. Here, a continuous step flow motion of the (111)-type facet in contact with the interface is observed.
Movie 3: Image series of the Pt NP presented in Figure 2G–J) at 600°C in an atmosphere containing 700 mbar O₂ plus 60 mbar H₂.
Case 3
The third platinum NP that was considered was oriented with (111) planes inclined towards the interface. Movie 4 below shows the image series acquired for this nanoparticle, at 600°C in an atmosphere containing 700 mbar O₂ plus 60 mbar H₂. In this case, the nanoparticle is observed to engage in redox chemistry-driven directional surface migration which is caused by restructuring at the interface.
Movie 4: Image series of the Pt NP presented in Figure 2K–N) at 600°C in an atmosphere containing 700 mbar O₂ plus 60 mbar H₂.
Retraction of H₂ and reformation of the oxidic SMSI overlayer
The next step for Frey and his fellow researchers was to switch the gas composition from a reactive feed back to a purely oxidizing environment by turning off the H₂ flow. This change in the gas composition resulted in the encapsulated state of the platinum NPs to be reestablished. In Figure 3 below, the morphological change of a Pt NP upon switching the gas from a redox-active environment to a purely oxidizing environment is presented. This switching of the gas composition led to the reformation of a classical particle overgrowth. It is seen that the nanoparticles first adopted a spherical morphology (Figure 3A–C). Then, as soon as H₂ was fully removed from the Nano-Reactor, the support material migrated onto the Pt NPs and the overlayer reformed (Figure 3D–F).

Figure 3: Image series depicting the morphological changes of a Pt NP observed when switching from a redox-active to purely oxidizing environment at 600°C. t0 is the time at which the H₂ flow was set to zero.
Conclusion
The aim of this paper was to reveal the working state of a catalyst via direct observation and to study possible synergistic interactions related to SMSI between metal nanoparticles and a reducible oxide support. The use of our Climate system enabled the researchers to capture in exceptional detail the dynamic and complex metal-support interactions of Pt–TiO₂ under reactive catalytic conditions. This is especially thanks to the advanced capacity of the system to handle pressures of up to 2 bar.
A key finding of this paper is that the stable configurations of static Pt particles exhibiting encapsulation were observed to exist either in pure H₂ (the classical SMSI state) or in pure O₂ (the nonclassical SMSI state), but not in an environment where both gases were simultaneously present. Indeed, the exposure to the redox-active environment led to the removal of the overlayer and the subsequent emergence of pronounced particle dynamics. Moreover, the in situ observations show that the particle restructuring and migration behavior is dependent on the relative orientation of the particle on the support, and therefore the configuration of the interface. Overall, these findings advance our comprehension of SMSI-induced encapsulation of metal nanoparticles, which can in turn help us better tune the chemisorption and catalytic properties of catalysts.

“The DENSsolutions Climate System allows us to reveal the so-far unseen: Looking at not only how the gas-phase is changed in the presence of a catalyst, but also studying how the interaction between gas-phase and catalyst leads to the emergence of catalytic function. Direct real-space observation is essential for our understanding of working catalysts and the development of new processes that are urgently needed in view of climate change and limited natural resources.”
Prof. Dr. Marc-Georg Willinger
Professor | Technical University of Munich
Original article:
Discover our Climate solution:
Discover more publications made possible by Climate:
Climate helps uncover phase coexistence and structural dynamics of redox metal catalysts
Using our Climate system, scientists are able to interrelate the atomic-scale structural dynamics of redox metal catalysts to their activity.