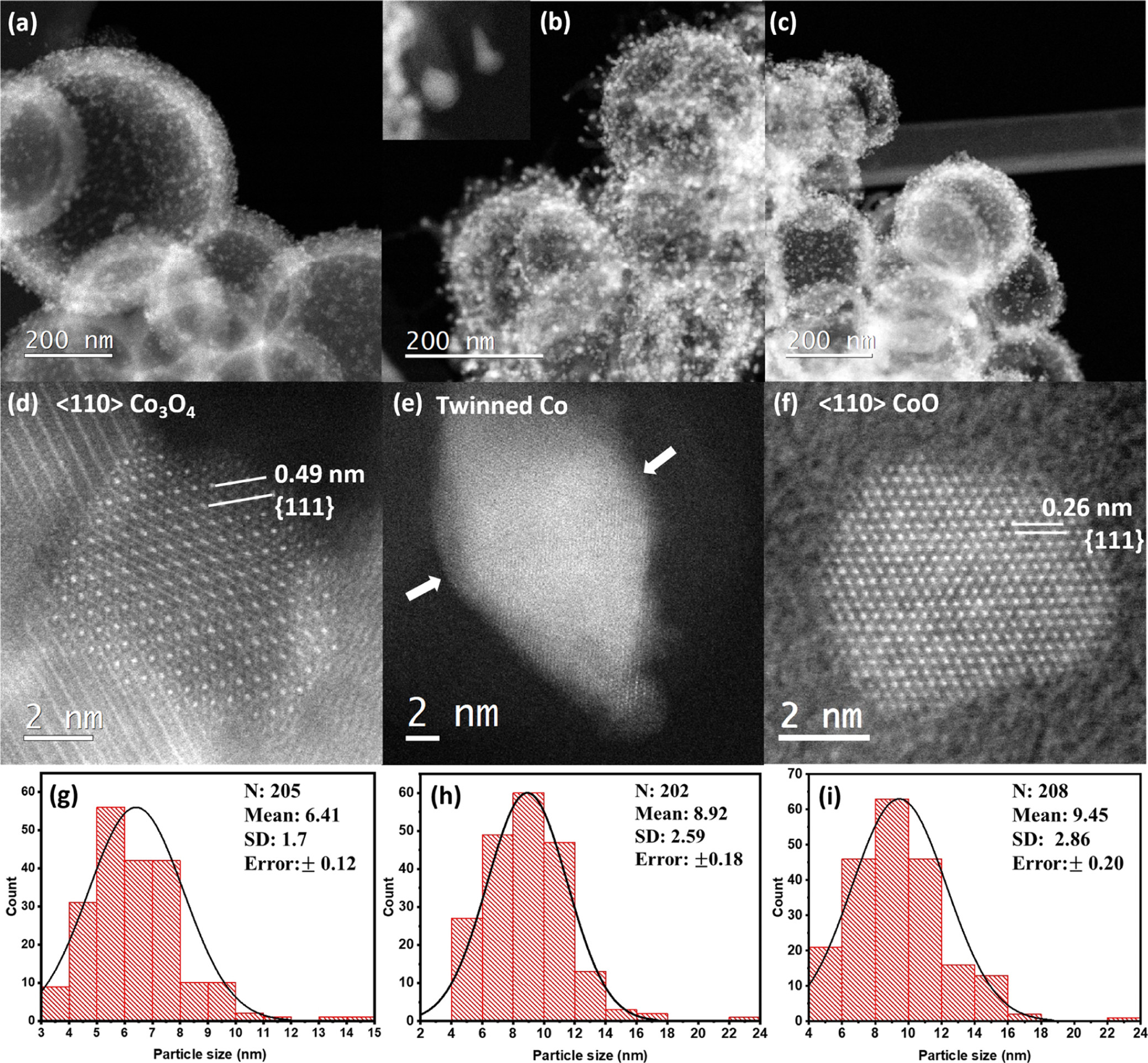
DENSsolutions successfully installs another Climate system in Japan, at JFCC

Top row – from left to right: Mr. Suzuki (Nano Tech Solutions), Mr. Anada (JFCC), Dr. Lars van der Wal (DENSsolutions) and Mr. Hirai (JEOL). Bottom row – from left to right: Mr. Fukunaga (JEOL), Mr. Jinbo (JEOL) and Mr. Hisada (JEOL).
We are proud to announce that DENSsolutions has installed another Climate system in Japan, at the Japan Fine Ceramics Center, located in Nagoya, a highly populated Japanese port city. In this article, we interview Dr. Satoshi Anada, Senior Researcher at the Nanostructures Research Lab in JFCC, to learn more about their microscopy facility, its research direction, as well as how our Climate system is advancing their research.
Can you tell me about Japan Fine Ceramics Center and its research and development initiatives?
“Japan Fine Ceramics Center (JFCC) was established back in 1985, with the goal of improving the quality of fine ceramics mainly through integrated testing and evaluation systems. JFCC has numerous business activities, one of which is the research and development (R&D) of materials, manufacturing technology and evaluation technology. Our R&D initiatives are focused on obtaining technological solutions to problems related to the environment, energy and safety. We have two main laboratories: 1) the Materials R&D Lab and 2) the Nanostructures Research Lab. The Materials R&D Lab focuses on the development of highly functional and novel materials (mainly ceramics) by improved process control, whereas the Nanostructures Research Lab focuses on the development and enhancement of state-of-the-art electron microscopy and related technologies. At the Nanostructures Research Lab, we have a high-end electron microscope – the JEOL JEM-ARM300F2 Grand ARM. This microscope enables us to observe samples at ultra-high spatial resolution with highly sensitive analysis over a wide range of accelerating voltages.”
What type of applications are the users at the Nanostructures Research Lab interested in using the Climate G+ system for?
“Users at the Nanostructures Research Lab are interested in applying the DENSsolutions Climate system to record operando TEM observations of battery and catalyst materials. We aim to understand where and how reactions take place, and which conditions enhance the performance of those materials. Moreover, we are interested in the electrochemical oxidation of materials in reaction with oxidants such as oxygen and hydrogen.”
What particular features of the DENSsolutions Climate G+ system attracted you to the system?
“In order to understand factors and mechanisms related to the performance of battery and catalyst materials, it is important to observe their reactions in the actual environments in which they are used. The Climate system has the ability to flexibly and rapidly adjust gas composition, temperature, flow and pressure, which enables us to observe our battery and catalyst materials under various experimental conditions. This is capability is particularly what attracted us to the solution.”
In your experience so far, how have you found working with the Climate G+ system?
“The preliminary processes including the assembly of the Climate Nano-Reactor and leak testing are quite straightforward, assisted by the well-established Climate manual and software. With the Climate system, we have been able to perform numerous experiments without running into any leakage issues. Moreover, we are particularly impressed with the stability of the system even at extremely high temperatures.”

Dr. Satoshi Anada
Senior Researcher | Japan Fine Ceramics Center
Dr. Satoshi Anada received his Ph.D. degree in Engineering, Material Science, from Osaka University. Previously, he was working as a Specially Appointed Assistant Professor in the Research Center for Ultra-High Voltage Electron Microscopy at Osaka University. Currently, Dr. Anada is working as a Senior Researcher in the Japan Fine Ceramics Center (JFCC). His research was focused on the electromagnetic analysis of functional materials and devices using transmission electron microscopy, and now particularly on different microscopic measurement informatics.
Discover Dr. Anada’s publications:
Learn more about Climate:
Discover publications made possible by Climate: