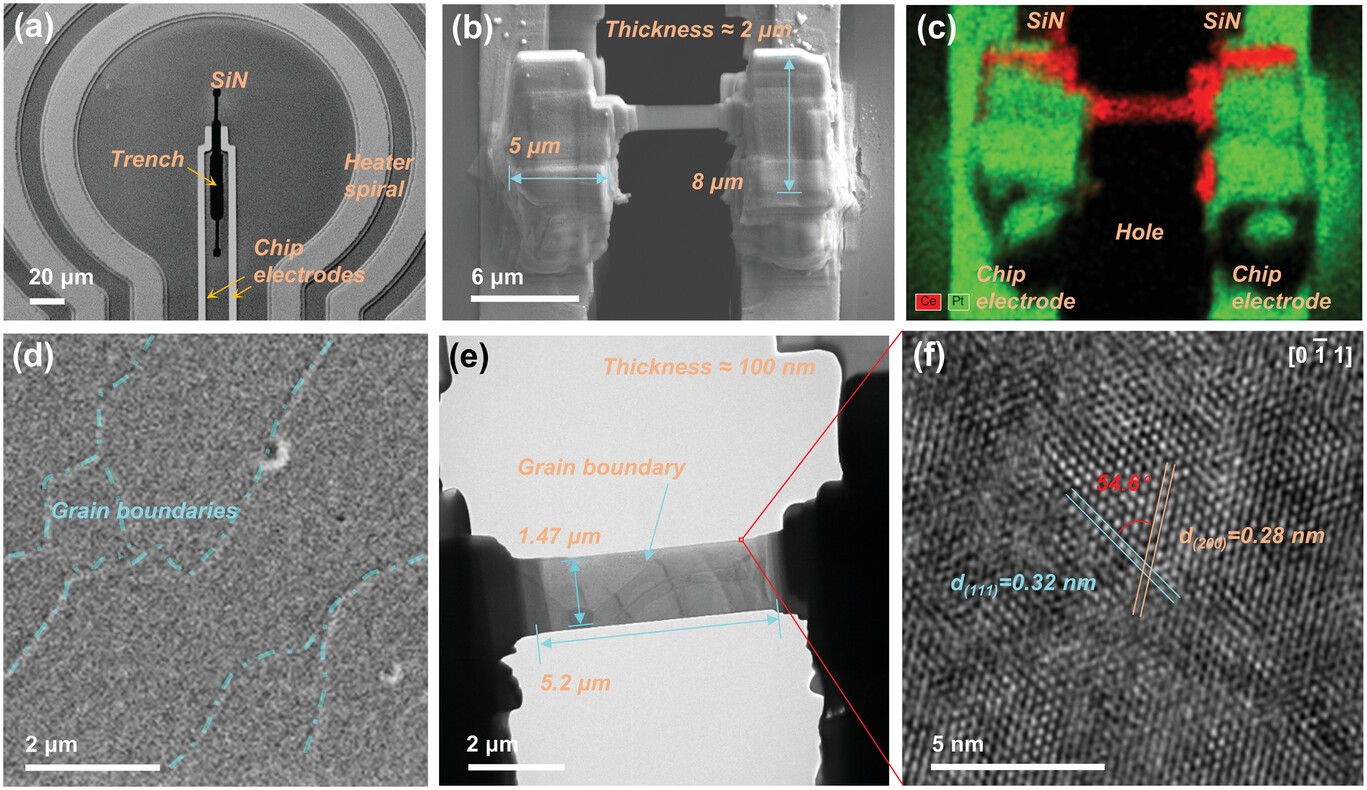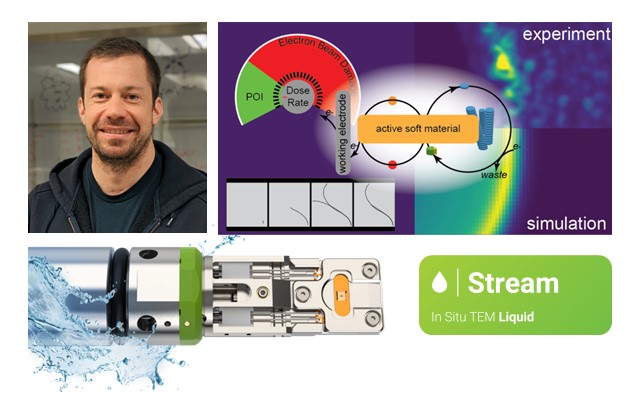
In situ LPEM: Illuminating the electrochemical nanoscale dynamics of active materials
Using the DENSsolutions Stream system, researchers take a magnified look at the nanoscale processes governing electrochemical activity in active molecular materials.
Original article by Gibson et al.

Active materials’ ability to interact with their environment in dynamic ways makes them invaluable across numerous fields, enhancing the functionality, efficiency and sustainability of various products and technologies that we use daily. This includes bio-sensors, flexible electronics, water purification and solar cells. Indeed, a thorough comprehension of the behavior of active materials under electrochemical conditions is crucial for their development. While substantial efforts have been made to understand the self-assembly mechanisms of biologically active materials, there is currently a large knowledge gap on how synthetic active materials behave. Traditional microscopy techniques often fall short in capturing the real-time dynamics of materials immersed in liquid environments. This is where liquid phase electron microscopy (LPEM) comes into play, offering a powerful solution to bridge this gap.
In a recent study published in the renowned journal of ACS Nano, a team of researchers from the University of California (UC) Irvine and the University of Massachusetts Boston employed the DENSsolutions Stream system to investigate the dynamics of electrochemically driven active materials. Impressively, they were able to capture single fiber dynamics at subsecond temporal resolution, as well as larger transitory fiber foci structures with nanoscale resolution. This research, involving our dear user at UC Irvine, Prof. Dr. Joe Patterson, is a major step forward in using electrochemical liquid EM to understand the dissipative self-assembly processes that generate active materials – a research space that remains largely unexplored. Notably, our Stream system played a vital role in enabling the visualization of these intricate electrochemical processes, providing key insights into the relationship between chemical kinetics and material dynamics.
Hierarchical evolution of fiber dynamics
The nanoscale self-assembly processes observed in this study involve the electrochemical oxidation of a free cysteine thiol precursor (CSH) molecule to its disulfide gelator form (CSSC) using the ferricyanide/ferrocyanide redox couple as a electrochemical catalyst. For the experiments, Wyeth Gibson and his fellow collaborators utilized the Stream Nano-Cell’s working electrode as the anode, which provided the driving force for the oxidation of ferrocyanide to ferricyanide and the follow-up oxidation of CSH to CSSC.
After capturing the dynamics of individual fibers under electrochemical stimulation near the electrode, the researchers were then able to capture the micrometer-scale hierarchical evolution of fiber clusters. As shown in the movie below, the fiber foci experience a maximum growth at 87 s and disassembly at 167 s. Evidently, the overall growth and shrinking of the fiber foci seem to loosely correspond with application and removal of electrochemical stimulus.
Movie 1: LPEM movie depicting the fiber foci growth and disassembly
Capturing fiber foci modification
The next step for the researchers was to study the active material’s dynamics in response to further electrochemical stimulus. They applied a current to the structures and activated the electron beam, which was maintained at a constant throughout the experiment. As shown in Movie 2 below, for the first 100 seconds, the fiber foci remained stable. At 100 s, a self-assembly growth front moved from left to right, causing the structures to grow and increase in contrast. At 200 s, a second growth front emerged as the first front reached the electrode boundary, spreading outward from the electrode in all directions. Between 400 and 600 seconds, the structures began to break down, shrink and decrease in contrast across the viewing window.
Movie 2: LPEM movie depicting the electrochemically driven fiber foci modification
Movie 3: LPEM movie depicting the fiber foci modification
Next, a regional segmentation analysis was performed in order to quantify the observed wave-like propagation of these self-assembly fronts. This is depicted in Movie 3, whereby the middle panel shows the segmented particles corresponding to the LPEM movie shown in the left panel. The graph in the video depicts the normalized change in segmented particle area over time for each region, with the segmented regions represented by blue (closest to the electrode), purple, red, and yellow (farthest from the electrode).
It is evident that the distance from the electrode affects the maximum structural density in a sequential manner. Notably, Dr. Gibson and his collaborators effectively demonstrate that the active material can be dynamically manipulated to form multiple growth fronts influenced by electrodes at different spatial locations.
Next, a regional segmentation analysis was performed in order to quantify the observed wave-like propagation of these self-assembly fronts. This is depicted in Movie 3, whereby the middle panel shows the segmented particles corresponding to the LPEM movie shown in the left panel. The graph in the video depicts the normalized change in segmented particle area over time for each region, with the segmented regions represented by blue (closest to the electrode), purple, red, and yellow (farthest from the electrode). It is evident that the distance from the electrode affects the maximum structural density in a sequential manner. Notably, Dr. Gibson and his collaborators effectively demonstrate that the active material can be dynamically manipulated to form multiple growth fronts influenced by electrodes at different spatial locations.
Movie 3: LPEM movie depicting the fiber foci modification
Integrating observations with simulations
To gain a clearer understanding and measure the structural transformation within the liquid cell, the researchers utilized structural dissimilarity (DSSIM) analysis on the electrochemical LPEM video. DSSIM analysis is a video processing technique that spatially and temporally quantifies structural changes occurring in a video. Importantly, by combining the LPEM data with kinetic simulations, they discovered that the formation of an active material can foster a local environment that boosts the pace of the self-assembly process, exhibiting an autocatalytic behavior.
Movie 4: DSSIM visualization and quantification of fiber foci dynamics
A pioneering electrochemistry study
Conclusively Prof. Dr. Joe Patterson and his fellow researchers performed a cutting-edge study, employing a combination of techniques including LPEM, electrochemical analysis, quantitative video analysis and kinetic simulations to explore a widely untapped research space – the self-assembly mechanisms in electrochemically fueled active materials. This innovative research highlights the crucial role of liquid electron microscopy in studying active materials, offering vital insights into the interplay between chemical kinetics and material behavior. We are certainly proud of the key role that our Stream system has played in bringing this research to fruition, and we look forward to the pioneering academic contributions that the Patterson Lab will continue to deliver.

“These were the most challenging liquid electron microscopy experiments I have ever performed, and the DENSsolutions Stream system was essential for getting them to work.”
Prof. Dr. Joe Patterson Professor | University of California Irvine
Original article:
Discover our Stream solution: