
Climate helps uncover phase coexistence and structural dynamics of redox metal catalysts
Using our Climate system, scientists are able to interrelate the atomic-scale structural dynamics of redox metal catalysts to their activity.
Original article by Xing Huang, Travis Jones, Alexey Fedorov, Ramzi Farra, Christophe Copéret, Robert Schlögl, and Marc-Georg Willinger
Metal catalysts have been extensively studied due to their critical role in industrial redox reactions. However, many gaps in research still remain, hampering the optimization of their design. Specifically, the behavior of metal catalysts under operating conditions and the relationship between structural dynamics and catalytic activity are still not fully understood. Indeed, an atomistic comprehension of the structure–activity relationship of working catalysts is essential for the optimization of their design.
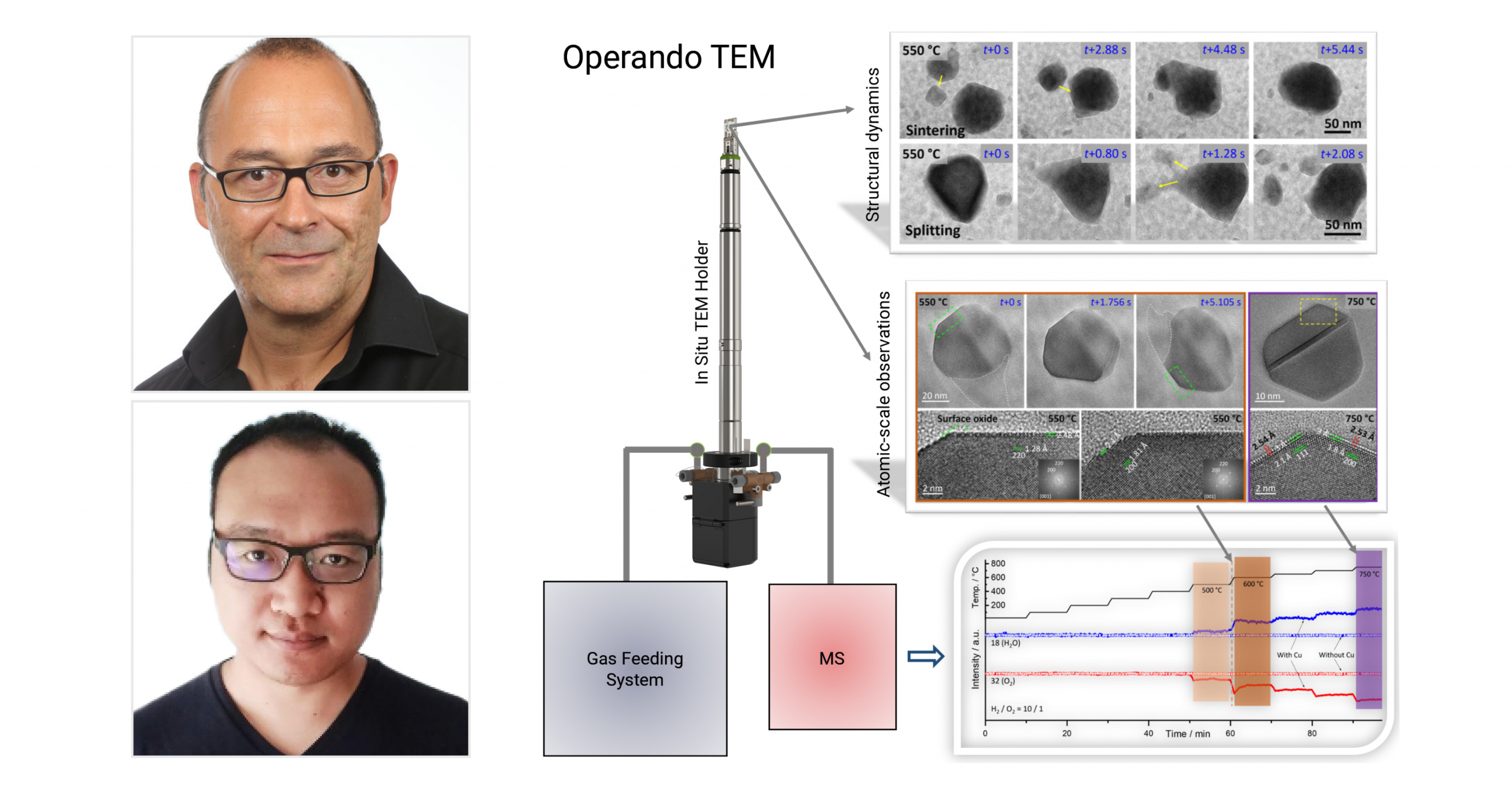
In their recently published paper, Dr. Marc-Georg Willinger from ETH Zurich, Dr. Xing Huang from Fuzhou University and fellow collaborators from the Fritz-Haber Institute of Max-Planck Society and the Max Planck Institute for Chemical Energy Conversion explore the phase coexistence and structural dynamics of redox metal catalysts. Using our Climate system, the researchers are able to achieve controlled gas-flow and imaging, obtaining atomic-level insights into the correlation between the structural and chemical dynamics and catalytic function. In light of today’s environmental challenges, the development of improved catalysts for more resource-efficient processes is becoming increasingly critical. Importantly, developing improved catalysts requires their direct observation during operating conditions. In this work, the authors have obtained atomic-scale insights into catalyst dynamics in various relevant redox reactions.
Redox reactions
Copper is a popular transition metal used as an active component in redox catalysts for many reactions, including CO₂ reduction and water gas shift reaction (WGSR). However, an atomistic description of the state of copper under redox conditions in these catalysts remains unrealized. In this publication, Dr. Willinger and his fellow collaborators present a detailed, high-resolution study of copper during the hydrogen oxidation reaction (HOR), revealing fundamentals of catalyst dynamics under reactive conditions. Beyond the elementary hydrogen oxidation reaction, the researchers extend the observed dynamic behavior to more relevant redox reactions and other metal catalysts. Specifically, they explore the redox dynamics of copper and palladium in the active state during methanol oxidation and methane oxidation reactions, respectively.
Hydrogen oxidation reaction on copper
Studying HOR offers the opportunity to obtain detailed atomistic insights into the reaction between the catalyst and the gas phase. HOR was chosen because it is the most elementary redox reaction and yields only water as a product, thereby reducing the complexity related to potential electron-beam-induced processes. In this study, the researchers systematically assess the effects of temperature and gas-phase conditions. Moreover, they explore how the chemical potential of the gas phase defines the phase, size, and shape of catalyst particles, driving the system into a nonequilibrium dynamic state during catalysis. Due to the simultaneous detection of catalytic conversion, they are able to relate directly the observed dynamics and surface structures to catalytic activity.
1) Redox dynamics and structural analysis of Cu
Copper nanoparticles of 50–200 nm were first loaded in the Climate Nano-Reactor. In situ TEM images of the copper nanoparticles were then recorded. It was found that the particles exhibit rich structural dynamics, which are associated with reconstruction and random motion, as well as particle sintering and red-ox induced splitting. The figure (A–L) and movie below depict these structural dynamics. Shown in M) is the integrated SAED pattern and corresponding radial intensity profile. The in situ SAED revealed dynamically appearing and disappearing diffraction spots, and confirmed the presence of metallic copper and Cu₂O as the sole oxide phase. The constant competition between oxide growth and reduction are reflected in the in situ SAED and the observed structural dynamics. Indeed, the structural dynamics are a consequence of chemical dynamics, characterized by phase coexistence and continuous interconversion between Cu⁰ and Cu₂O. The high-resolution imaging in Figure 1N) confirms this, showing a metallic head coherently interfacing with an oxide tail.

Figure 1: The redox dynamics and structural analysis of Cu. A–L) show the in situ TEM observations of catalyst reshaping (A–D), sintering (E–H), and splitting (I–L). In M) the integrated SAED pattern and corresponding radial intensity profile are shown. N) shows the HRTEM image of a nanoparticle containing a metallic head coherently interfacing with an oxide tail.
Movie 1: The redox dynamics of copper showing catalyst reshaping, sintering and splitting.
2) Effects of temperature and gas-phase composition
The authors then sought out to explore the effect of temperature on the observed redox dynamics. First, the temperature was decreased from 500 to 300°C, while maintaining a H₂/O₂ ratio of 10/1. During the temperature decrease, the researchers observed the growth of oxide dendrites, which reflects the increasing oxidation potential. Simultaneously, due to the slower kinetics of the redox reaction at lower temperatures, a reduction of the structural dynamics was observed. During heating from 300 to 750 °C, the system passes through a regime of increased dynamics (550 °C) that are characterized by translational motion and restructuring due to oxide growth and reduction, until it finally reaches a state that is less dynamic and dominated by metallic copper at 750°C. This is shown in the figure below (A–D). The reconstructed HRTEM images taken at 300 and 750°C are shown in 2E) and 2F), respectively. Next, integral SAED (Figure 2G,H) was performed to investigate phase analysis, revealing the relation between phase composition and temperature. It has been previously demonstrated that the trend in the oxide content reflects the decreasing chemical potential of oxygen with increasing temperature. However, the authors observe a notable exception of this general trend at around 550 °C, which is mostly due to the effect of water that is produced at substantial rate and contributes to the redox dynamics.

Figure 2: Chemical potential versus structural dynamics of Cu. A–D) In situ TEM observation of dynamics at 300–750 °C and a H₂/O₂ ratio of 10/1. E,F) show the reconstructed HRTEM images taken at 300 and 750 °C. G) and H) show the normalized radial profiles extracted from the integrated SAED patterns and subsequent radial intensity profile, respectively. I–L) In situ observation of copper dynamics during decreasing H₂/O₂ ratio from 10/1 to 5/1 at 500 °C.
Next, the researchers explored the influence of gas-phase composition on the structural dynamics, gradually decreasing the H₂/O₂ ratio from 10/1 to 5/1 at 500 °C. It was found that the relative increase of the oxygen partial pressure leads to a transformation of initially spherical nanoparticles into elongated particles with a head–tail structure. This is depicted in Figure 2I–L above. At the same time, the average particle size declines due to an increased rate of particle splitting, until a new size regime and dynamic equilibrium is established. Conclusively, the real-time observations under varying gas-phase composition and temperature show a clear effect of the gas-phase chemical potential on the average particle size. The in situ observations show clearly that redox dynamics make particles mobile, thereby considerably increasing the rate of sintering as compared to thermal sintering; yet the sintering under redox conditions is balanced by particle splitting, such that a certain size distribution is established as a function of reaction conditions.
“Controlled gas-flow and imaging – coupled with on-line mass spectroscopic analysis of the gas-phase composition as enabled by the Climate system – is essential for studies on the behavior of active catalyst and allows us to correlate observed structural and chemical dynamics to catalytic function.” – Dr. Marc-Georg Willinger, ETH Zurich
3) Relation between structural dynamics and catalytic activity
After investigating the gas-phase and temperature-induced dynamic processes, the researchers then sought out to explore the relationship between the observed structural dynamics and catalytic activity. The MS data is presented in Figure 3A) below, showing the formation of water and simultaneous consumption of oxygen. This ultimately confirms the catalytic activity of copper. A notable increase in water production and oxygen consumption is observed between 500 and 600°C, which is also the same range in which the intense structural dynamics occurred. In Figure 3B–D), the sequential HRTEM images of particle reshaping/restructuring at 550°C is presented (H₂/O₂ ratio of 10/1). This is also shown in the movie below. Although challenging, the researchers were still able to capture the thin oxide monolayer existing on the surface of the metallic portion of the particles (see Figure 3E,F). Interestingly, the surface oxide layer is observed even at 750°C. The structural features of the monolayer oxide imaged on various facets can be observed in Figure 3G,H).

Figure 3: Relation between structural dynamics of Cu and catalytic activity. A) shows the MS data collected at varied temperatures. B–D) show the sequential HRTEM images of particle reshaping/restructuring at 550°C. E,F) show the enlarged HRTEM images of the areas indicated by dashed rectangles in (B) and (D). G) shows an HRTEM image, and in H) an enlarged HRTEM image of the area indicated by the dotted rectangle in (G).
Movie 2: HRTEM movie showing particle reshaping and restructuring at 550°C
Methanol oxidation reaction on copper
After investigating the particle dynamics for hydrogen oxidation on copper, the researchers then set out to assess the generality of the phenomena described above. They first investigated the state of copper under conditions of methanol oxidation, a catalytic reaction that is relevant to industrial synthesis of formaldehyde. The figure below shows in situ TEM images of copper nanoparticles recorded at 600 and 500°C (Figure 4A–C and 4E–G), respectively. The dynamic behavior observed involves reshaping, sintering, and splitting of particles, similar to what was observed in the case of hydrogen oxidation. A shift to a more oxidized state with decreasing temperature was observed and verified by in situ SAED (Figure 4D,H). The redox dynamics are most pronounced at around 500°C under the chosen 1:1 ratio of MeOH and O₂.

Figure 4: Structural dynamics of Cu in methanol oxidation reaction. A–H) show TEM images and SAED patterns of Cu recorded in situ during methanol oxidation at 600 °C (A–D) and at 500 °C (E–H), respectively.
Methane oxidation reaction on palladium
Next, they investigated methane oxidation on palladium, a transition metal that is much harder to oxidize than copper. In the figure below, the structural dynamics related to catalytic activity in methane oxidation on palladium is presented. As in the case of copper, structural dynamics evolve when palladium is driven toward the Pd/PdO phase boundary. In an ~2:1 ratio of CH₄ and O₂, the catalyst remains relatively static at 350°C and shows coexistence of Pd and PdO as evinced by the in situ SAED. The system evolves to a highly dynamic state at 550°C. The MS data recorded simultaneously with TEM observation reveal a pronounced formation of CO₂ and consumption of CH₄ and O₂ under these conditions (see Figure 5I).

Figure 5: Structural dynamics related to catalytic activity in methane oxidation on Pd. A–H) In situ TEM images and SAED patterns of Pd recorded during methane oxidation at 350°C (A–D) and 550°C (E–H), respectively. I) shows the MS data recorded during in situ TEM observation of Pd in methane oxidation.
Conclusion
Via the above in situ studies of copper and palladium catalysts using our Climate system, the researchers show that catalytic activity goes hand-in-hand with redox processes of the metal catalyst. This paper evinces that the associated dynamics sensitively depend on reaction temperature and gas-phase composition. Importantly, only direct observation could reveal the interplay between metal and oxide phases and relate it to the onset of catalytic activity. This is precisely where our advanced in situ solutions come into play, enabling the direct observation of phenomena while it occurs. We are proud of the role that our Climate system has played in making this research possible and strive to continue enabling groundbreaking research in the future.

“The DENSsolutions Climate System allows us to reveal the so-far unseen: Looking at not only how the gas-phase is changed in the presence of a catalyst, but also studying how the interaction between gas-phase and catalyst leads to the emergence of catalytic function. Direct real-space observation is essential for our understanding of working catalysts and the development of new processes that are urgently needed in view of climate change and limited natural resources.”
Dr. Marc-Georg Willinger
Group Leader | ETH Zurich
Original article:
Discover our Climate solution:
Discover more publications made possible by Climate:

DENSsolutions’ Climate system takes home the Microscopy Today 2021 Innovation Award
DENSsolutions becomes a consecutive two-time winner of the Microscopy Today Innovation Awards. This year, our Climate system is recognized as one of the 10 most game-changing microscopy innovations of 2021.

