Via the unique on-chip microfluidic channel of the DENSsolutions Stream system, researchers were able to create a highly controlled chemical environment for visualizing the nanoscale metallic electrodeposition of copper crystals.
Original article by Cheng et al.
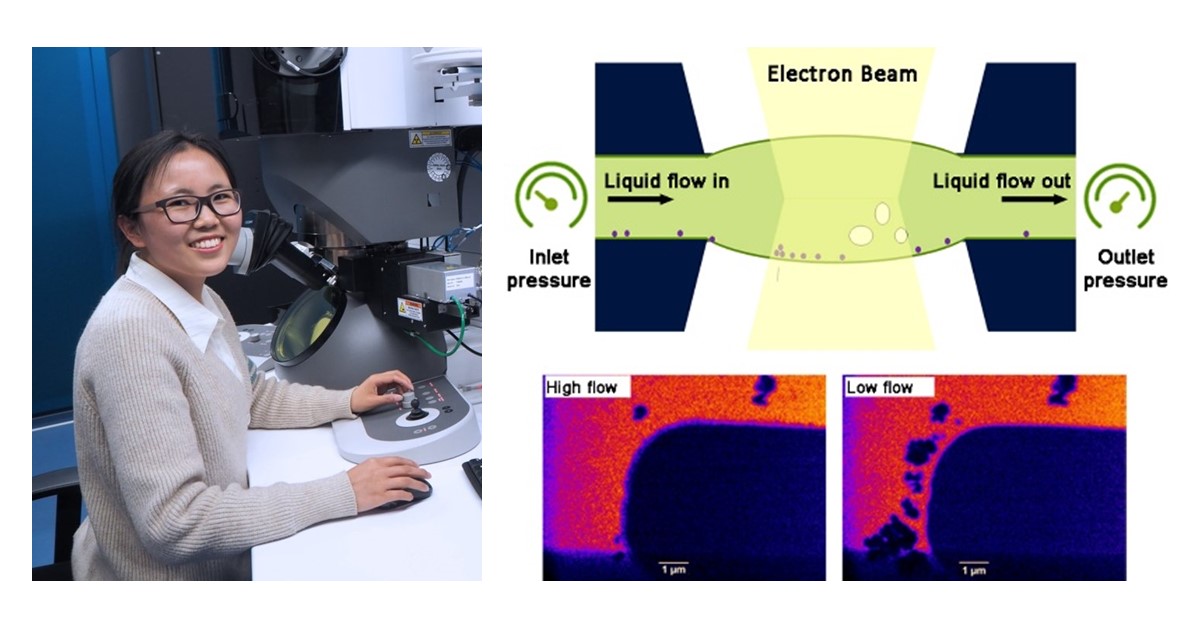
Liquid phase transmission electron microscopy (LPTEM) enables the observation of time-resolved dynamics in liquid state at high spatial resolution. The technique has gained exponential popularity over the last decade, and has contributed greatly to a wide range of fields, including materials science, chemistry and life science. With LPEM, researchers can explore the dynamical evolution of key materials and uncover fundamental insights into nucleation and growth. Only in recent years have researchers been able to control the chemical environment within an in situ LPEM experiment, owing to the award-winning innovation that is the DENSsolutions Stream system. In a recent publication, researchers including Dr. Ningyan Cheng from Anhui University utilized the Stream system to visualize the metallic electrodeposition of copper crystals in a highly controlled chemical environment. This was made possible due to the unique on-chip flow channel of Stream, which enables numerous advantages such as the ability to flush away beam-induced species, explore flow-dependent liquid dynamics and easily change electrolyte composition.
On-chip microfluidic channel
The core of the DENSsolutions Stream system is our patented Nano-Cell, which consists of a top and bottom chip, together forming a sealed compartment that enables users to safely perform liquid experiments inside the TEM. The bottom chip contains spacers, an integrated liquid inlet, flow channel and an outlet. Via pressure-based pumps, a liquid sample can be driven from the inlet through the field of view and then through the outlet. This process is demonstrated in the video below. Importantly, users can independently control the pressure at the inlet and outlet of the Nano-Cell, and therefore the absolute pressure in the microfluidic channel. This then enables full control over the liquid flow rate within the cell.
Movie 1: Animation depicting the microfluidic channel of the Stream Nano-Cell
Efficient liquid flow
Before observing any liquid phenomena in the TEM, Dr. Cheng and her fellow collaborators first had to ensure that the flow was efficient and well-controlled. To do this, the researchers first assembled a dry Nano-Cell. The flow was then initiated by turning on the pressure-based pump, while keeping all imaging parameters constant. After 30 seconds, the imaging contrast changed abruptly, implying that the liquid had definitely flowed into the Nano-Cell. This process is shown in the video below. The time taken to completely fill the Nano-Cell ranges anywhere from tens of seconds to just 3 minutes when a flow rate of 8 μl/min is applied.
Movie 2: In situ TEM movie showing the liquid flow into the Nano-Cell in just 30 seconds
Removal of beam-induced species
A key benefit of controlling the liquid flow within an LPEM experiment is the ability to remove beam-induced particles. The researchers first generated particles by increasing the electron flux on purpose through changing the spot size from 5 to 1. The process of removing the beam-induced species in this experiment is detailed in the video below, with the direction of the flow going from top to bottom. As soon as the flow was cut off at 6.4s, the particles started to form and grow on the membrane. The flow was then switched on again at 11.7 seconds, which is when the particles that were stuck to the membrane started to peel off and move from the top to the bottom area in the field of view. It took just 2 minutes to fully flush away the particles, which is a reproducible process. The direction of the particles’ movement is the same as the direction of the flow (top to bottom), confirming the effectiveness and power of the liquid flow control.
Movie 3: Removal of beam-induced species via liquid flow control
Capturing flow-dependent liquid dynamics
The next step for the researchers was to explore the effect of the flow rate on the electrochemical copper crystallization and dissolution processes in real time. They first observed the effect of using a higher flow rate of 1.4 μl/min on the Cu deposition and dissolution processes, which showed to be reversible. The protocol included the initial electrode cleaning, deposition (−0.9 V, 10 s), dissolution (+0.4 V, 15 s) and repeating the process for 4 cycles. As demonstrated in the video below, the researchers found that uniform copper deposition can be obtained at a higher liquid flow rate (~1.4 μl/min), whereas at a lower liquid flow rate (0.1 μl/min), the growth of copper dendrites was observed.
Movie 4: Copper electrodeposition at a flow rate of 1.4 μl/min (left) and 0.1 μl/min (right)
Changing electrolyte composition
Besides exploring the effects of altering the flow rate, a major point of interest in this study was observing the effect of adding foreign ions, such as phosphates, on the electrodeposition. Such additives can affect the electrochemically deposited crystals by, for example, changing the nuclei structures. The researchers first studied the electrodeposition of copper from a pure CuSO₄ aqueous solution. In this case, no obvious dendritic morphology was observed and instead only granules were formed (see below Figure 1, Left).
After the experiment, the electrolyte in the sample source was replaced by a mixture of CuSO₄ and KH₂PO₄ solution. The liquid was kept flowing with a flow rate of 3 μl/min for 15 min, which enabled the researchers to directly study the electrolyte effect on the depositions in the same liquid cell by excluding all the uncertainties during different cell assembly. Contrastingly in this case, copper dendrites were observed to grow and the addition of H₂PO₄− ions in the electrolyte led to the formation of Cu-phosphate complexes (see Figure 1, Right). These results further confirm the importance of being able to modulate the electrolyte composition, and demonstrate the effectiveness of the environment control that Stream enables.
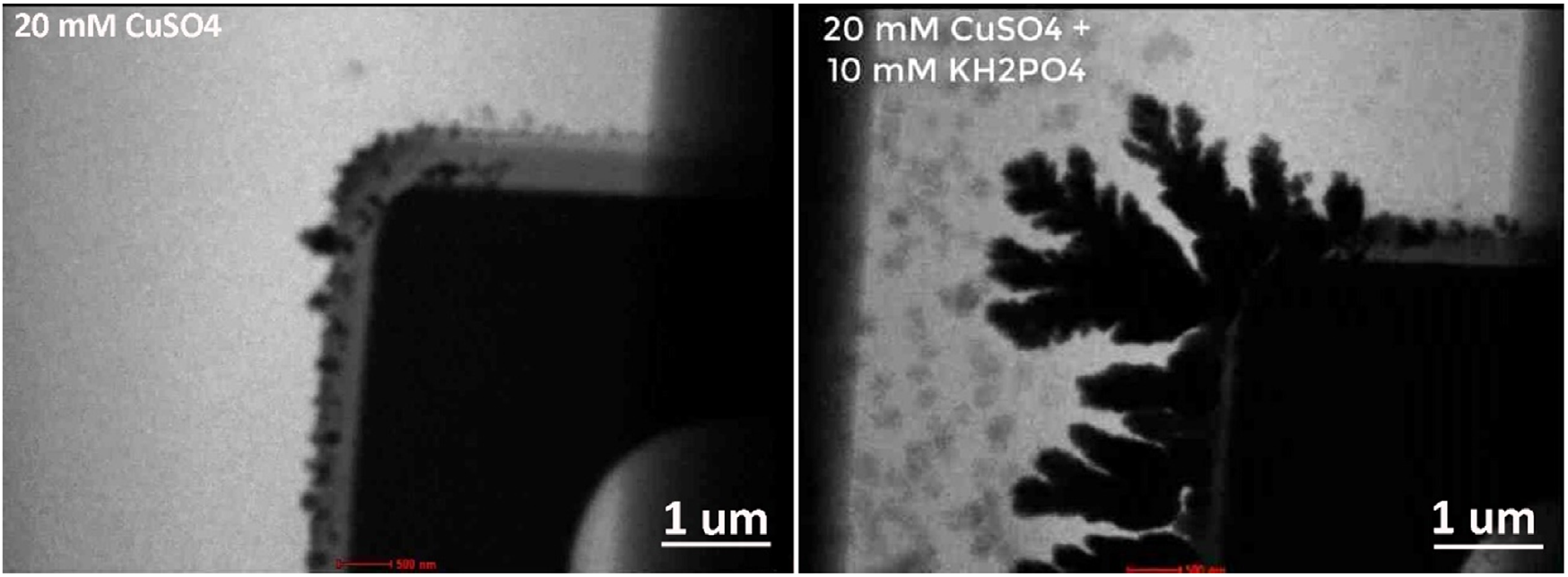
Figure 1: The effect of phosphate addition on Cu electrodeposition
Exploiting electrode design to alter the chemical environment
When studying Cu electrodeposition in the previous experiment, the researchers saw that dendrite formation can be further promoted by the in situ addition of foreign ions, such as phosphates. In order to confirm the generality of this technique, they also took a look at Zn electrodeposition in an aqueous solution of ZnSO₄. The figure below shows the total growth of the zinc layer at b) a lower potential of −0.9 V (versus Pt) in the first 10 seconds, and c) a higher potential of −1.1 V (versus Pt) in the next 10 seconds. In d) the total growth of the Zn depositions in the first 20 seconds is shown. The researchers observed in the first 10 seconds (−0.9 V) that the deposition on the inner edge is rougher compared to the outer edge. In the following growth at −1.1 V, dendritic depositions are nucleated and grown on the previous outer edge, while no further growth can be observed in the inner edge. This experiment demonstrates that the special electrode design of Stream enables the exploration of rich liquid dynamics within different chemical environments.

Figure 2: Zinc electrodeposition in b) the first 10 seconds with a potential of −0.9 V and c) in the next 10 seconds with a potential of −1.1 V. d) shows the total Zn growth in the 20 seconds.
Conclusion
Through this study, it is shown that Stream’s distinctive ability to enable liquid flow control opens the doors for researchers to truly alter the chemical environment within the liquid cell. By controlling the liquid flow, a user can flush away beam-induced species, explore flow-dependent liquid dynamics and easily change electrolyte composition. Moreover, the unique design of the electrodes in the Stream system allows researchers to explore complex liquid dynamics within different chemical environments within the same liquid cell. Importantly, the direct observations made by Cheng et al. not only provide new insights into understanding the nucleation and growth, but also give guidelines for the design and synthesis of desired nanostructures for specific applications, such as high performance electrocatalysis for energy conversion and electrodes for secondary batteries.

Image of Dr. Ningyan Cheng from Max-Planck-Institut für Eisenforschung GmbH
“The DENSsolutions Stream System not only provides a useful means to study a wide range of dynamics in solution, but also enables systematic studies of the effect of the chemical environment on the corresponding reactions through precise control of the flow rate, liquid composition and other significant parameters.”
Prof. Dr. Ningyan Cheng Associate Professor | Anhui University
Original article:
Discover our Stream solution:
Discover more publications made possible by Stream:
Climate helps uncover phase coexistence and structural dynamics of redox metal catalysts

