Our partnership with the EPSRC/Jeol Centre for Liquid Phase Electron Microscopy at UCL, London
DENSsolutions LPEM systems enable advances in Life Science

Dr. Lorena Ruiz-Perez (left) and Prof. Guiseppe Battaglia (right)
On the 12th of November, DENSsolutions in cooperation with UCL and Quantum Design UK will be holding a Stream workshop at the EPRSC/Jeol Centre for Liquid Phase Electron Microscopy (LPEM) facility at UCL, London. At the LPEM facility, opened in 2017, Dr. Lorena Ruiz-Perez uses the DENSsolutions Liquid In Situ solutions to characterise soft organic nanomaterials via TEM imaging. In this article, we take a look at the LPEM research that Ruiz-Perez is doing within the Molecular Bionics lab.
Molecular Bionics
The goal of the group is to mimic specific biological functions and/or introduce operations that do not exist in nature by engineering bionic units made of polymers. This goal is achieved by a multidisciplinary team of chemists, physicists, mathematicians, engineers and biologists.
The LTEM team at the Molecular Bionics group is formed by Prof. Guiseppe Battaglia, director of the facility, Dr. Lorena Ruiz-Perez, manager of the facility. Cesare de Pace and Gabriele Marchello are PhD students involved in the experimental development of LTEM and LTEM image analysis respectively.
Inside the group, Dr. Lorena Ruiz-Perez has been using the DENSsolutions Ocean system to work mainly on two different projects.
Polymer assemblies
For the first project, she has been using the system to investigate soft matter polymer assemblies. As we have shown in one of our earlier articles, these assemblies have the potential to be used for targeted drug delivery inside the human body. These kinds of assemblies have been well studied using Cryogenic electron microscopy (cryo-EM). One of the main advantages of employing LPEM is that it allows us to gain new insights into the dynamic behaviour of these assemblies within a liquid that were not possible using images of the vitrified, i.e. frozen sample. In liquid, you can observe for instance the fluctuation of the polymer assembly membranes and hence investigate significant mechanical properties of the soft materials.
Proteins dynamic behaviour
Their second project involves investigating the dynamic behaviour of proteins in liquid. These proteins move by the so-called ‘Brownian motion’. The group wants to understand the structure of the proteins inside their native environment. While the protein is moving in water, they can capture many different profiles in order to reconstruct a 3D image of the protein structure. There is a minimum frame amount needed for the reconstruction, so the time component becomes fundamental in these in-situ studies. The investigation aims to create a library of proteins, like the RCSB PDB, with information on dynamic processes which can complement the information already supplied by the well established cryo-EM technique. Their first results, studying ferritin proteins, were presented at Manchester 2019*.




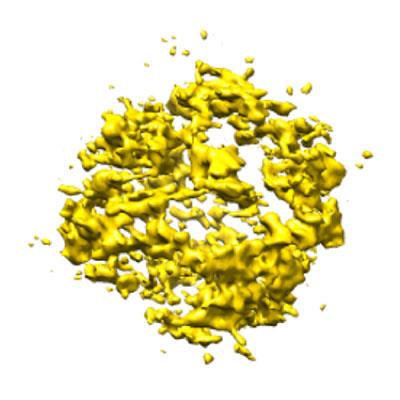
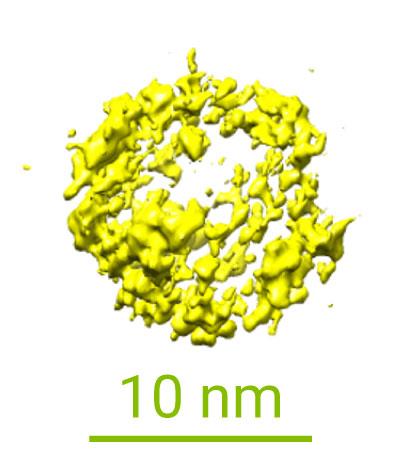
Proteins play a pivotal role in our physiological conditions and associated diseases. A deeper understanding of the kinetics governing the mechanistic behaviour of proteins in liquid media can lead to big improvements in drug design and ultimately in general healthcare.
*This manuscript is currently being updated with long molecular dynamics simulations of ferritin in solution.
The new Stream system
Now the group is advancing to the DENSsolutions Stream system, allowing them to do new kinds of experiments. The big advantage of the Stream system is that it can control the bulging of the viewing windows and therefore the liquid thickness. Controlling the bulging is essential for creating reproducible results. In previous LPEM in situ systems, the window bulging could differ between experiments, thus preventing experiment reproducibility.
Now with the Stream system, the bulging can be adjusted precisely for each new experiment, guaranteeing the same level of bulging and, therefore, consistent results. Controlling the liquid thickness is also important to achieve high contrast in organic and biological materials. The liquid thickness can be reduced up to the equilibrium where you have the highest possible resolution combined with a thick enough layer to have a realistic sample environment.
Learn more about our Stream system:
Discover publications in which our Ocean system was used: