Uppsala University in Sweden expands its TEM capabilities using DENSsolutions In Situ systems
A DENSsolutions Wildfire double tilt (DT) system with a biasing expansion has been installed at the Uppsala University, Sweden.


“It will be mainly used by the solid state chemistry group in order to investigate temperature behavior of alloys ”
Lars Riekehr – senior research engineer from the Ångström Laboratory in the Department of Engineering Sciences at Uppsala University.
Applications
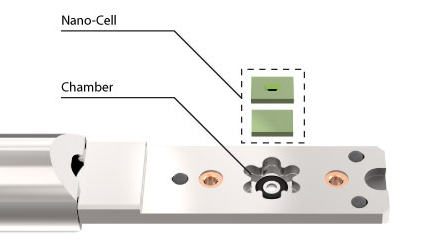
The Wildfire DT system will be used by the group to research the phase transitions in metals and solar cells.
“After the installation the system was directly tested using solar cell samples. The researchers wanted to see how chemical inhomogeneities in the absorber layer would behave upon heating.”
In particular, Riekehr is pleased with certain features of the Wildfire system, such as the extra lateral shift on heating, the bulging and the stability. Additionally, the Biasing expansion will allow for precise operating voltage control.
He added that he was looking forward to the prospect of being able to offer in situ experiment results to anyone in the department who might need them.
Thank you for reading
To learn more about our Wildfire system: