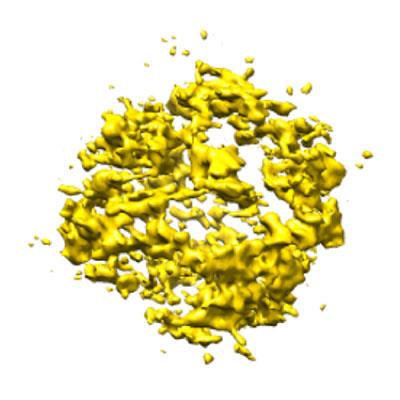
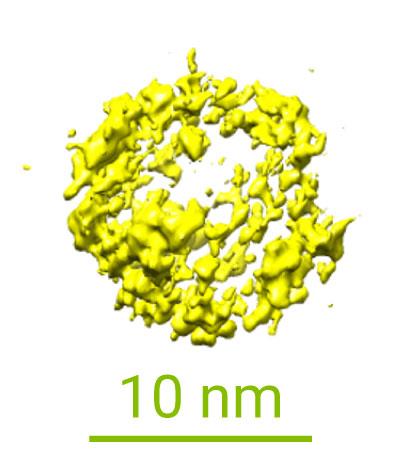
First Visualisation of Crystal Growth from Organic Molecules
Scientists characterise how flufenamic acid, a COX inhibitor, which is used in multiple industries, forms crystals from a liquid solution
Original article by Jennifer Cookman, Victoria Hamilton, Louise S. Price, Simon R. Hall and Ursel Bangert. Published in Nanoscale, issue 7, 2020.


Experimental Firsts
“Using LCEM (liquid cell electron microscopy) it has been possible for the first time to capture early-stage nucleation events of organic molecular crystals used as APIs (active pharmaceutical ingredients),” said Dr Jennifer Cookman from the Bernal Institute at the University of Limerick and lead researcher for this study.
“The importance of this method is that we can begin to understand how one crystal structure forms over another and, even more importantly, how these early-stage nucleation events manifest. We can also compare/contrast with classical nucleation theory and other crystal growth theories.”
The molecule studied, flufenamic acid, is a COX-inhibitor that acts as an anti-inflammatory. With increased study of this molecule, which is used in the pharmaceutical industry, researchers can potentially fine-tune its action as a medicinal drug and reduce its side-effects.
What’s more, the molecular crystalline state is widely used across many industries; including electronics and agrochemicals. This research represents the first steps to analysing molecular crystal growth of not just flufenamic acid, but other molecules with implications for improvements in other industries.
A 55-second video of flufenamic acid crystal growth at a scale of 0.5 micrometers. This video is remarkable as it shows the entire process of crystal nucleation, from a blank screen through nucleation to crystals. A good region to examine is the central point just above the 0.5 μm scale. A crystal forms here and grows without getting cluttered by other crystals.
Techniques and Methods
The Ocean LPEM system’s unique ability to perform transmission electron microscopy in a liquid ethanol environment was essential for this research. Dr Cookman adds that by “using the DENSsolutions Ocean holder we were able to introduce an undersaturated liquid solution of the API to be visualised in the TEM protected from the high vacuum environment of the TEM.” Then crystal growth was induced by illuminating the sample with an electron beam which provided the energy needed to prompt the nucleation process.
In situ microscopy far exceeds previous ex situ observations as the team could produce live footage of each stage of crystal growth. Additionally, the in situ technique enabled the visualisation of the nucleation of flufenamic acid molecules in a working environment, a.k.a. ethanol. Performing this analysis in a liquid environment as opposed to a vacuum helped to meaningfully ascertain where and how different physical arrangements of crystal structures occur.
“This work brings focus to the use of electron microscopy and in particular in situ TEM equipment for characterisation,” added Dr Cookman. “That can be of utmost importance to the pharmaceutical industry and also in interim characterisation in pharmaceutical crystal research.”


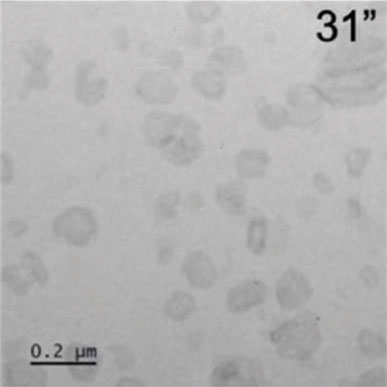


Micrographs showing crystals forming from flufenamic acid and growing into hexagonal crystals. A 0.2-micrometer scale is used for reference.
Wider Importance
It is at this earliest stage of growth that molecules can exhibit polymorphism; crystal structures that are composed of the same molecules but have different physical arrangements. Understanding how different crystal structures form from the same molecule type is desirable for research that needs nanoscale precision. For example, crystals are commonly used in medicines as a way to deliver active chemicals.
The antiretroviral drug, Ritonavir, which is used to treat HIV/AIDS, was pulled from circulation after it was found to contain a polymorphed version of the active drug. The polymorphed form was less biologically active and did not work as intended. Understanding these early steps in crystal growth is key to fine-tuning processes such as drug delivery.
Future Research
Advances in film technology and TEM allowed for direct observation of the nucleation process and this research represents the potential progress in the field of crystallisation. The results indicate that, with more research, scientists can discern the initial phases of crystal growth. This new technique opens up the field of TEM to visualising other crystallisation pathways, interrogate nucleation mechanisms, and explore new innovations.
The research was part of an EU Horizon 2020 FET-Open project named MagnaPharm which focuses on the magnetic control of polymorphism in pharmaceutical compounds. The team, which includes Dr Ursel Bangert and Dr Simon Hall, intend to continue to characterise flufenamic acid and different growth outcomes under different concentrations.
Learn more about our latest LPEM system ‘Stream’:
Discover more publications made possible by our Ocean system:
Read the original article: